
Radiocarbon nomenclature can be a significant stumbling block to researchers applying radiocarbon to their work for the first time. A multitude of units are currently in use, with the unit of choice varying by scientific discipline. Different AMS labs also report their data in different units (e.g. one lab reports data in percent modern, while another lab reports data in D14C, Δ14C, Fraction modern, or standard radiocarbon age). Some labs may even give a calibrated age or date range on their data reports. Data misinterpretation can occur when the data recipient either misunderstands what the number represents (e.g. standard radiocarbon age vs. calibrated age), or performs unit conversions incorrectly (e.g. (Δ14C/1000)+1≠Fraction modern).
Radiocarbon nomenclature has been discussed at length in book chapters and publications. Here we offer a condensed list of resources and a brief explanation of common units and unit conversions. If you have a specific question you'd like guidance on, please utilize our discussion forum.
Book chapters:
- The most recent review of radiocarbon nomenclature can be found in Chapter 3 of the book, Radiocarbon and Climate Change.
- Chapter 6 in, Biophysio-Chemical Processes Involving Natural Nonliving Organic Matter in Environmental Systems, offers additional discussion of radiocarbon nomenclature in the context of soil organic matter cycling.
Prominent publications covering nomenclature and data correction:
- The original formal definition of units was first published by Stuiver and Polach, 1977.
- Donahue, 1990 discusses the specifics of correcting samples for isotopic fractionation using a δ13C value measured apart from the AMS.
- Are you conducting elevated-CO2 experiments? Torn and Southon, 2001, explains how to correct your radiocarbon values for isotopic fractionation under these circumstances.
- Reimer et al., 2004, discusses nomenclature and fractionation correction conventions associated with dating of post-bomb samples.
Common sources of confusion
Historical perspective: Willard F. Libby, the developer of radiocarbon dating, did not have access to high precision accelerator mass spectrometers. When Libby and others were first calculating the half-life of radiocarbon, they had to rely on somewhat rudimentary scintillation counting instruments and measurement of known age samples. Therefore, the original half-life of 5,568 years that they settled on was slightly off. As the number of radiocarbon measurements grew and instrumentation advanced, a new value for the half-life was chosen by consensus at the Fifth Radiocarbon Dating Conference meeting at Cambridge in 1962. This value of 5,730 years is the currently accepted half-life of radiocarbon, and is referred to as the "Cambridge half-life". The previously used incorrect value of 5,568 years is referred to as the "Libby half-life".
Why are both half-lives still in use when we know one is incorrect? The consensus of the Fifth Radiocarbon Dating Conference was that, "... further experiments may lead to an even more reliable result." Therefore officially switching from one half-life to another was postponed (Godwin, 1962). Dendrochronology was still a nacent science at this point, and researchers were just beginning to understand that radiocarbon production in the atmosphere can vary over time due to the influence of sun spot activity (Stuiver, 1961), and fossil fuel combustion (Suess, 1955). Other causes of variation in atmospheric radiocarbon production were discovered in subsequent years.
A standard radiocarbon age, sometimes abbreviated as RCA, gives the relative radiocarbon abundance of a sample, expressed in years before present. The calculation of RCA takes into account only the radioactive decay of the radiocarbon isotope and does not account for variation in atmospheric radiocarbon production over time. RCA is expressed in units of years before present (BP), with year zero being 1950.
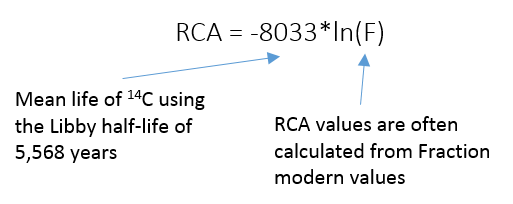
The date of 1950 was chosen to honor Libby's publication of the first list of radiocarbon dates, but also conveniently falls before the bomb curve. Because year zero is 1950, any RCA calculated for a sample more recent than 1950 will be negative. These negative RCA values have no real meaning, and should not be used for any type of data analysis. Most labs that report RCA will list samples more recent than 1950 as modern, or >modern. RCA values aren't of much use for scientists working with samples from open reservoirs (e.g. soil, atmosphere, etc.) but can be used for calibration of a true calendar age for samples from closed systems (e.g. tree rings, archaeological artifacts, plant macrofossils). Following historical tradition, RCA values are still published in the journal, Radiocarbon.
AMS laboratories vary in the exact mathematical formula used to calculate Fraction modern (F, Fm, or F14C) due to differences in instrumental setup and data reducing software. Equation 2 given below is the most basic form of the equation and the derivation of the parameters and coefficients.
Equation 2. Calculation of Fraction modern with definition of variables.
Other units may be calculated from a F value if the year of AMS measurement is known. Conversion equations are given below. Automated conversion options are coming soon!
Definition of variables not defined in text above:
yr = year of radiocarbon measurement
1950 = year zero
8033 = Mean life of 14C using the Libby half-life of 5.568 years
8267 = Mean life of 14C using the Cambridge half-life of 5,730 years
Pm = percent modern
